|
Sugars
In the end, our cane mills are
about producing syrup. Sugars are the principal
ingredients by weight of syrup and confer protection
against spoilage, provide sweetness and body, and
contribute to color if caramelized. Therefore, sugars
merit some mention on this site. First, a general
description of carbohydrates is given. Second, relevant
carbohydrates are described. Third, the conversion of
selected carbohydrates is outlined. Finally, why this
matters to syrup makers is addressed.
Sugars are ubiquitous, diverse,
and abundant in plants, where they serve a myriad of
functions. The simplest of sugars are strings of 3 to 7
carbon atoms. Carbon forms 4 bonds, and the two bonds
remaining (after the formation of bonds with neighboring
carbons) are used to bond with a hydrogen atom on one
"side" and a hydroxyl (OH) moiety on the other.
Simple sugars, therefore, are characterized as having the
ratio of C:H:O = 1:2:1; in short, sugars are
carbohydrates. In the following narrative, I have placed
in bold the main carbohydrates that syrup makers are
concerned with. Glucose (= dextrose),
fructose (= levulose, fruit sugar), and sucrose (=
table sugar, cane sugar, beet sugar) are the main sugars
in cane juice, and starch is the starting point for
making corn syrup (which is blended with cane syrup by
some syrup makers).
Glucose and fructose have six
carbon atoms. Because each of the carbon atoms along the
string can have the H on the "left" or on the
"right" if a flat projection of the string is
made, many different sugars have six carbons, and, of
course, the same goes for sugars of other sizes.
Simple sugars can linked together
to form larger molecules, such as sucrose.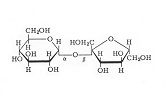 Note that sucrose is made from one glucose
molecule (the six-membered ring on the left) joined to a
fructose molecule. This figure also makes it easy to
visualize, as discussed above, how so many different
sugars can result simply by "flipping" the H and
the OH. Lactose (milk sugar) and maltose (discussed
later) are other examples of sugars that are formed from 2
units of 6-carbon sugars. You have probably noticed that
the trivial name of many sugars ends in -ose. Note that sucrose is made from one glucose
molecule (the six-membered ring on the left) joined to a
fructose molecule. This figure also makes it easy to
visualize, as discussed above, how so many different
sugars can result simply by "flipping" the H and
the OH. Lactose (milk sugar) and maltose (discussed
later) are other examples of sugars that are formed from 2
units of 6-carbon sugars. You have probably noticed that
the trivial name of many sugars ends in -ose.
Finally, very large carbohydrates
can be made by linking up to tens of thousands of simple
sugars to form long chains. Cellulose-cotton fiber is
almost pure cellulose-is an extremely abundant resource
and is used to make a variety of products such as paper
and plastics. Cellulose is a chain of glucose molecules
joined from the # 1 carbon on one to the # 4 carbon on the
next. Starch is another large carbohydrate made
exclusively from glucose. Starch consists of two kinds of
chains, though. One kind of chain (amylose) is linear
(again, the # 1 and # 4 are linked to form the chain). The
other kind of chain (amylopectin) has a 1>4 backbone,
but also has branches; amylopectin is very similar to
glycogen, which is the main readily available food reserve
stored in animals ranging from oysters to humans.
Two important points arise. The
physical and biological properties of apparently very
similar carbohydrates are vastly different. Take cellulose
and amylose from the preceding paragraph. Both are simply
chains of glucose units connected between the #1 and # 4
carbons. However, the orientation of the connecting bond
is different. Cellulose is very strong with a tensile
strength exceeding steel; starch has no strength. Starch
forms a gel in hot water, as when we make gravy; cellulose
is unphased by water, as we observe each time we wash
cotton clothes. Starch from wheat, rice, and corn are the
foundation of a good diet; we cannot digest cellulose.
Sucrose and starch can be broken
down using simple chemical means. Sucrose is broken down
into glucose and fructose by heating with acid. Starch is
also broken down into fragments by acid treatment;
depending on the duration, the strength of the acid and
the temperature, breakdown can be complete, all the way to
individual glucose units. Less complete breakdown of
starch yields, among other products of various lengths,
maltose (a fragment of two glucose units). Some types of
corn syrup are prepared by partial or complete acid
hydrolysis of corn starch. There are many different corn
syrup formulations. I chromatographed one source of corn
syrup used by many syrup makers in South Georgia, and it
was pure glucose. One the other hand, I chromatographed a
sample of syrup blend produced in South Georgia from a
different brand of corn syrup and it could not have been
pure glucose.
Carbohydrates can be converted
from one to another using enzymes (proteins that
specifically speed certain reactions.) A commonly used
enzyme is an invertase obtained from yeast. This enzyme
catalyzes the breakdown of sucrose into glucose and
fructose. (Glucose and fructose are thus invert sugars
because a physical property, rotation of plane-polarized
light, has been inverted [changed from plus to minus] by
the conversion of sucrose to the two 6-carbon sugars). If
the treatment is too short or if the conditions (e.g.,
amount of invertase) are not optimum, all the sucrose may
not be broken down. A second commonly used enzyme is one
of the amylases, which partially break down starch.
Amylases are used to manufacture some corn syrups and to
prevent gelling of sorghum syrup. Finally, another enzyme,
an isomerase, converts a portion of glucose to fructose.
Use of this enzyme permits the production of High-Fructose
Corn Syrup (after the starch has been converted to
glucose). Corn syrup is, of course, a cheap sweetner and
various formulations find their way into many foods such
as breakfast cereals, canned fruit, ketchup, soft drinks .
. . . As you have noticed, the trivial names of enzymes
end in -ase.
What does all this mean to a
syrup maker?
- Sugaring. Of the three
sugars in cane juice, sucrose is the least soluble
(i.e., syrup will "hold" less sucrose before
it forms crystals). Although some sugaring is
acceptable, it has two drawbacks (esthetics to some
consumers and potential spoilage of the remainder of
the syrup). Fortunately for the syrup maker, some
sucrose is chemically degraded to glucose and fructose
by heating because the juice is slightly acidic (pH ~
5.2) [see section above]. (An early extension bulletin
indicated that the longer heating time in kettles is
an advantage because the syrup contains less sucrose,
if all else is equal.) There are three remedies for
sugaring under the control of the syrup maker. First,
he or she can choose a variety of cane
that is less likely to form sugar in syrup. This is a very
effective strategy to produce excellent excellent
syrup. Second, he or she can add corn syrup to
the sugar-cane syrup, effectively diluting the
sucrose. Many syrup makers opt for this choice.
Whether to blend or not to blend is a matter of
personal preference, of course, and some prefer the
milder taste of the blend anyhow. Third, and finally,
the sucrose content of the syrup can be diminished by
the use of invertase (see Walton CF, EK Ventre 1935
How to prevent sugaring of sugarcane sirup. USDA
Circular). Walton and Ventre suggested taking the
juice to semi-syrup (20 º Baumé) before treatment.
As I understand it, invertase is considered a
"processing aid," not an ingredient, and
therefore does not need to be listed on the label.
Fructose is the most soluble of
the three sugars and is often chosen in food manufacture
for that reason (e.g., to prevent "sandiness" in
ice cream).
Interestingly, honey bees are
faced with the same problem as syrup makers. Nectar, like
cane juice, is mostly sucrose, glucose, and fructose.
Honey bees inject invertase into the nectar as it is
converted to honey. Thus, honey is mainly glucose and
fructose. Even so, some nectars, like that produced by the
mustard family, contain so much glucose that the honey
crystallizes quickly. On the other hand, tupelo nectar has
so much fructose that honey produced from it never
crystallizes.
-
Spoilage. Of course, the first
line of defense against spoilage is sterilization. A
second potential line is the addition of a
"preservative" such as benzoate. Again, this is
a matter of personal preference, but note that we accept
benzoate in products such as soft drinks. However, the
sugar composition may also play a role. A primary
historical means of preventing the growth of spoilage
organisms is to deny the organisms adequate water. This
goal is accomplished directly by drying the product such
as fruit slices or by "drawing" the water out
with usually salt. The relevant comparative
physical-chemical parameter to measure concentration in
this context is osmolality (osm; not osmolarity), which in
this case is the ratio of sugar molecules to water
molecules. What all this means theoretically is that for a
syrup of given sugar content (weight of sugar per volume
of syrup), a sucrose syrup would be the easiest for the
spoilage organism to thrive in.
Another property of a solution
that depends on osmolality is elevation of boiling point.
For a typical cane syrup, this value is about 12-13 º F
above the boiling point of water, or about 225 º F along
the coastal areas. As indicated in the discussion above,
this value will depend on which sugar (and other components plus deviations from
ideality) in the syrup predominates. (If sucrose
predominates, a lower boiling point would be expected, and
if the 6-C sugars predominate, a higher boiling point
would be expected.) As a general rule, thermometers, which
provide a continuous reading, are used in evaporators
whereas hydrometers are used in kettles, as discussed
below.
-
Thickness. The viscosity, or
resistance to flow, comes into play in two different ways.
First, some syrup makers rely on viscosity (i.e., flaking
off a dipper) to determine when the syrup is finished.
Second, some people have a preference for thicker or
thinner syrups. The least viscose sugar is fructose, with
glucose being slightly more viscose. Sucrose is, by far,
the most viscose (the relative viscosity at room
temperature is almost twice as high for sucrose).
Ironically, a syrup made of sucrose will be thicker than a
syrup made of fructose (or glucose) even when the latter
contains more sugar by weight! These facts, along with the
sharp temperature dependence of viscosity, explain the
difficulty of judging when the syrup is done by using
"flaking." On the other hand, the densities of
solutions made from different sugars at the same
concentration (weight per volume) vary by less than 1 %,
attesting to the utility of a hydrometer. Density, like
viscosity, is affected by temperature, however. For
example, a desirable density for finished cane syrup is
38.5 to 39 º Baumé at 70º F. This same syrup would test
at 34.5 º Baumé at 210 ºF. (Thanks to McCalip and
Walton for these figures from an article in USDA Bulletin
1370, 1925).
-
Sweetness. Fructose is the
sweetest.
|