|
Metals
I have given some thought to the
effects of different traditional processing surfaces on
the metal contents of sugar cane syrup, and an opinion has
attached itself to me. Here, I will set down the reasons
for my interests in metals, and provide some values for
iron, copper, and zinc in syrup, all being the facts that
support my opinion. Development of this perspective has
been a two-year personal odyssey, which I could not have
made alone. Bruce Smith assayed the metals. Ken Christison
has offered encouragement, provided for syrup samples to
be sent to me, and chased down important information. For
her part, my wife, Nedra, took me in when I was 24 and
finished rearing me; without that, I likely would have
made a different trip. I thank them all, but do not share
with them any of the blame for mistakes.
Because of the nostalgic grip
that my grandfather's
sugar-mill yard has on me, I
often mused wistfully about setting up a replica of this
operation. Reality, however, dictated otherwise. On the
one hand, I have a consuming and you-might-say pesky job
that admits of too little free time. On the other hand,
prospects for retirement seemed dim as my father had a
disabling stroke at 54, his brother died at 58, and his
father died at 45. And, each of the four of my father's
mother's brothers (Jerry, George, Newt, and Noah) died in
his forties. Thus, it was pretty well established that the
Sutton men, and some Outlaws, didn't live long, and I saw
no reason that I should expect to be an exception.
When my son, Will, moved from
without the umbrella of my health insurance coverage, he
sought other coverage. Obtaining new individual insurance
required that Will have a physical examination, which
revealed iron overload. In brief, Will was diagnosed with
hemochromatosis and the fatigue that we had attributed to
his medical-school regimen was explained now, in part, by
disease. Despite its being the most common genetic
disorder of Americans, hemochromatosis is not a household
word and it seems that too few physicians appropriate
proportionate attention to this illness. Hemochromatosis
is deadly serious and will cause just about whatever will
ail a person (e.g., 14% lifetime incidence of hepatic
cancer). Fortunately, hemochromatosis is usually easily
treated by periodic phlebotomy; if detected early and
treated, the length and quality of life are normal. As a
relevant aside, Will's disease, as implied, like most
hemochromatosis in the U.S., is hereditary.
Hemochromatosis can be acquired, however, from abnormally
high iron intake (e.g., through beer brewed in cast-iron
kettles).
Detection of disease is but one
small payoff of biological research that your taxes
support. For a small price (less than two hours of
machine-shop time!) and a small inconvenience (mailing a
cheek smear), single-nucleotide substitutions of the HFE
gene responsible for hereditary hemochromatosis will be
determined. In brief, Will is a homozygote for the most
common and implicated mutation (C282Y/C282Y) of the gene
product. My daughter Elizabeth also has exactly the same
genetic makeup, but as a menstruating person, her iron
never became elevated. My wife Nedra and I are both
heterozygotes, a condition with which little risk is
associated, and both of us monitor the relevant blood-iron
parameters. Interestingly, my sister is a H63D
heterozygote and my mother was homozygous for the wild-type
allele. That means, of course, that my father was a
compound heterozygote and his health condition probably
related to his genes. Based on these and other analyses,
the parsimonious explanation is that my father's H63D
allele was inherited from his father. The summary
conclusion is that I divorced the Grim Reaper and turned
my attention to building a syrup factory for my retirement
(. . . all the while religiously taking a statin, a beta-blocker,
and various nutriceuticals). As my goal was to be
authentic, I preferred to cook in cast iron.
This paragraph briefly addresses
with a minimum of jargon how genetic hemochromatosis is
diagnosed; those not interested should skip to the next paragraph. Genes, which function in concert with the
environment in the appropriate place and time, are the
essence of an individual. Most genes in humans are present
in two copies, one obtained from the mother and one from
the father. Genes may exist in slightly different forms,
so the two copies may be alike or they may be just a
little different. Because of these different forms of the
same gene, individuals can be distinguished by examining
several genes. The information in genes, for the most
part, is encoded by a string of nucleotides of four
different kinds, which, for shorthand, are designated A,
T, C and G. A gene is characterized, therefore, by its
sequence or order of its, say, 1000, nucleotides. Thus, a
gene sequence may be ATTATCCGAGC . . . . . A powerful and
widely used technique, the polymerase chain reaction (PCR),
permits one to selectively duplicate thousands of times
any sequence that lies between two short target sequences.
Specifically, by knowing the general sequence of the HFE
gene, any portion of the gene can be amplified for
sequence analysis. As a test of a protocol designed to
teach our biology undergraduates theory and practice of
PCR, my colleague and collaborator Lloyd Epstein collected
my sloughed-off cheek cells and "fished out" a
portion of my HFE gene from the nominal 35,000 genes in
those cells. After amplification by PCR, he turned the
sample over to another molecular biologist, Steve Miller,
who analyzed 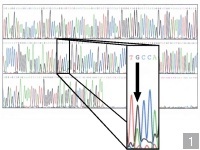 the
sequence (Slide
1). An examination of the sequence-start at the upper
left-shows the first position to be occupied by a single
red peak, which corresponds to a T here. The second
position is occupied by a single black peak, which
corresponds to a G, and the next two peaks are green,
corresponding to As. Since PCR amplified both copies of my
HFE genes, the single peak in each position indicates that
the nucleotide in that position is the same in both
copies, a situation that is generally true for all
positions. In one position, however (exploded view, Slide
1), there are two peaks-one corresponding to a G and
one to an A, meaning that the gene copy I inherited from
one of my parents is different from that inherited from
the other. In a normal person, only G would be present; in
a carrier, both A and G are present, and in a susceptible
person, both are A. Since my mother contained only G in
this position, I must have inherited the A in this
position from my father. The gene form I donated to both
of my children was the defective one containing A, as did
my spouse. Thus, both children have only A in that
position and are susceptible to genetic (or hereditary)
hemochromatosis. the
sequence (Slide
1). An examination of the sequence-start at the upper
left-shows the first position to be occupied by a single
red peak, which corresponds to a T here. The second
position is occupied by a single black peak, which
corresponds to a G, and the next two peaks are green,
corresponding to As. Since PCR amplified both copies of my
HFE genes, the single peak in each position indicates that
the nucleotide in that position is the same in both
copies, a situation that is generally true for all
positions. In one position, however (exploded view, Slide
1), there are two peaks-one corresponding to a G and
one to an A, meaning that the gene copy I inherited from
one of my parents is different from that inherited from
the other. In a normal person, only G would be present; in
a carrier, both A and G are present, and in a susceptible
person, both are A. Since my mother contained only G in
this position, I must have inherited the A in this
position from my father. The gene form I donated to both
of my children was the defective one containing A, as did
my spouse. Thus, both children have only A in that
position and are susceptible to genetic (or hereditary)
hemochromatosis.
It is well known that iron
dissolves from cookware into acidic foods, and it is also
known that diet can be a component of iron overload. The
long cooking times for processing sugar cane syrup in a
cast-iron kettle obviously raised questions about the iron
content of the syrup. Therefore, I became interested in
learning the iron content of syrup cooked in kettles, but
also considered cooking in copper, as copper evaporators
were also used. With Ken's help, I learned that the Food Code of the U.S. Department of Health and Human
Services proscribes the use of copper cooking surfaces for
juices with a pH below 6. The pH of sugar cane juice is
about 5.3, or 5-fold more acidic than pH 6. (pH, or
potential of Hydrogen, is the negative logarithm (base 10)
of the molar concentration of H+ [or hydronium ion], which
means that a small change in pH is a big change in
acidity.) Thus, copper went on my list to measure, along
with zinc (because galvanized iron is also proscribed).
(There are many factors that
control the absorption of an ingested ion like iron,
however, so how to figure in diet is at best problematic,
as I see it, except in extreme cases. As an example,
dietary iron from animal sources (coordinated with heme)
is relatively readily taken up; dietary iron from plant
sources (chelated to phytates) is only sparingly
available. Dietary interactions also come into
play-alcohol enhances uptake, as does orange juice
(because Vitamin C reduces the ferric ion to the ferrous
ion, which is the uptake species).
In the foregoing, I described why
syrup making interests me, which is a continuation of my
introduction. I also described why metals are of
interest to me. Now, I present a condensed version of the
findings (Table 1) in a self-explanatory format.
|
Table
1
The
concentrations of copper, iron, and zinc in sugar cane
syrup and sugar cane-syrup blends prepared
by the batch method in antique cast iron kettles (n
= 14) or by continuous evaporation in baffled linear
evaporators constructed of copper (n = 3). In both
cases, heat was provided by flame to the bottom of
the vessel. Metal concentrations were determined
using atomic absorption on a Perkin Elmer Zeeman
5100 furnace. Differences in metal contents as a
function of processing equipment were not
significant (P < 0.1). Cadmium was not detected
(< 10 ppb) in any of the samples.
|
| Metal |
Processing
Equipment |
Concentration
mg/100g
(x ± sd) |
Reference
Values1 mg/100g |
RDA
mg |
| Sorghum
Syrup |
Maple
Syrup |
| Copper |
Cast
Iron |
0.05
± 0.05 |
0.13 |
0.07 |
0.4
- 4 |
| Copper |
0.12
± 0.07 |
| Iron |
Cast
Iron |
5.60
± 11.90 |
3.80 |
1.20 |
6
- 15 |
| Copper |
6.30
± 9.50 |
| Zinc |
Cast
Iron |
1.14
± 1.20 |
0.41 |
4.16 |
5
- 19 |
| Copper |
1.25
± 0.70 |
|
1 The
reference values and the RDAs are taken from the
Nutrient Database, Release 13, of the United States
Department of Agriculture. This database does not
contain values for sugar cane syrup or sugar cane-syrup blends.
|
|