|
Crystallization
in Sugar-cane and Sorghum Syrups
As succinctly put by Bhandari &
Hartel (Journal of Food Science, 67: 1797-1802), the control
of crystallization of sugars is a major concern, being
important to the texture (e.g., of some candies) and
appearance (e.g., icing) of foods. In some foods, such as
honey, crystals are perceived as a natural and favorable
attribute in some cultures, such as Brazil's, and as a
defect in other cultures, such as ours. Yet, in other
cultures, such as parts of Europe, deliberate and controlled
crystal formation ("creamed
honey") is widely practiced. Generally, however,
crystals in syrups are thought undesirable, and indeed, may
negatively affect storage because the remaining liquid
is less concentrated. Still, in my opinion, it is important
to note, as seen by the example of honey, that markets might
respond to a non-liquid spread with the taste of sugar-cane
syrup or of sorghum syrup.
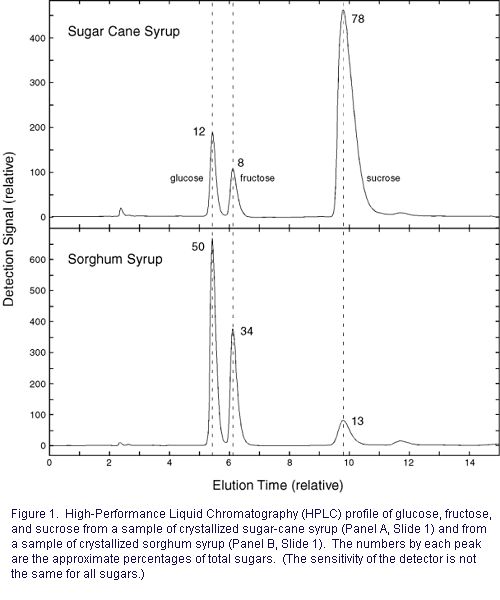
Crystal formation in sugar cane
syrup is common and crystal formation occurs some in sorghum
syrup as well (Panel A and B, respectively, Slide
1).
Prevention of crystal formation in sugar-cane syrup is theoretically
straightforward if in practice lacking details:
conversion of sucrose to its constituent monosaccharides,
glucose and fructose, will eliminate crystallization. This
conversion may be effected by use of invertase or by acid +
heat treatment. (The efficacy of invertase is discussed elsewhere;
use of lemon juice during processing by one Mississippi
sugar-cane syrup producer is known.) Consistently, the
crystallized sugar-cane syrup sample (Panel A, Slide
1) had
a high sucrose content (Fig. 1). (Sucrose
is the least soluble of the three major sugars.) As
indicated above, in all probability, this syrup would not
have crystallized had the sucrose content been reduced.
In some cases, reduction of the
sucrose content by use of invertase has been advocated by
extension personnel as a means to prevent sugaring in
sorghum syrup. These sources recommend using half as much
invertase as necessary for sugar-cane syrup.
Crystallization can be a complex
process, and crystals form in sorghum syrup that has low
sucrose content, too (Fig. 1). In such a sample, invertase
would not be effective, of course. Glucose is the most
abundant sugar in this sample, and glucose crystallization
is the main foundation for formation of creamed honey.
(Essentially, the only sugars in honey are glucose and
fructose. Honey high in glucose, such as that produced from
certain floral sources like mustard, are the best for making
creamed honey, and honey high in fructose, such as tupelo,
could probably not be crystallized under the same
conditions.) Unfortunately, there are no turnkey methods for
conversion of glucose to fructose in syrup (although the
concepts are in place to convert part of glucose to
fructose, depending on the starting ratio of the two
sugars.) The reader should note that I do not have any
direct experience in producing sorghum syrup before putting
a value on my thoughts for preventing crystallization if
it is a problem: (a) determine whether sucrose is a
cause of crystallization, or simply use invertase as a
prophylactic. (b) avoid seeding. Crystals grow on
"seeds," small surfaces, such as tiny crystals
themselves. It is common practice to open liquid syrup and
note that it crystallizes in days because seed crystals
form as syrup concentrates on the container, especially near
the spout, and forms seeds. When these seeds mix with the
syrup, there is a cascading effect. (c) avoid storage under
conditions that promote crystallization. That is, avoid
time-proven empirical methods for making creamed
honey. (d) reduce the brix, if feasible.
I mention again in closing that I
do not have specific expertise in the prevention of
crystallization in sorghum syrup, and that these suggestions
simply stem from my general knowledge of chemistry. I would
be delighted to incorporate other thoughts into this essay,
and am particularly seeking solid information that I could
use to update this essay.
|